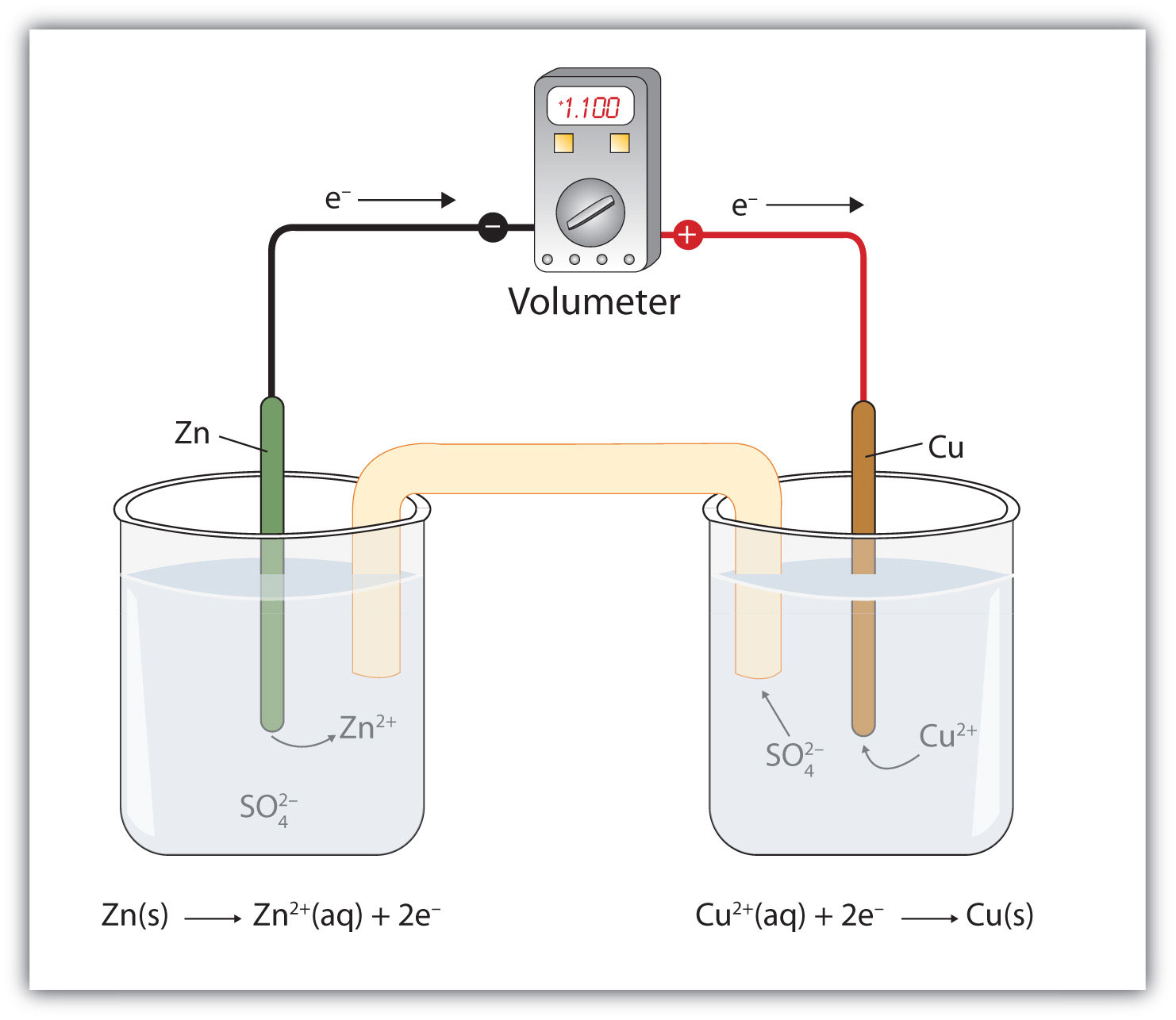
battery half cell
How cells work lithium batteries are an example of an electrolytic cell. an electrolytic cell is an electrochemical cell in which a non-spontaneous reaction is. 2012-05-22 ams battery & fc lectures - battery (michele p. for hubert g.).ppt p. 30 electrochemistry basics - electrochemical cells & ion transport. Electrochemical half cells and reactions used electrochemical cells are batteries. a battery contains chemical substances waiting to react; the reaction between.
Lead-acid battery : principles of operation john denker * contents 1  if we add the two half-cell reactions together, we get the full-cell discharge reaction. Cact homepage half cell reactions skills to develop explain chemical reactions for each electrode of a battery or galvanic cell. use notations to depict a electrode.. Lithium battery chemistry comprise a number of cell designs that use lithium as the anode. lithium is these cells represent over half of all battery sales..